NEWSNews

From May 16 to 18, 2024, the "23rd China Biologics Congress (CBioPC2024)", co-hosted by the China Vaccine Industry Association, the Biologics Branch of the Chinese Preventive Medicine Association, the Vaccine Professional Committee of the China Pharmaceutical Biotechnology Association, and the Biologics Professional Committee of the Chinese Society for Microbiology, was grandly held in Guangzhou. More than 8,000 attendees participated in the conference, including representatives from government departments at all levels, regulatory authorities, industry associations, experts and scholars from domestic and foreign biomedical fields and scientific research institutions, as well as enterprise representatives.
The theme of this congress is "Biomedical Innovation and Public Health Security". Guided by the spirit of the 20th National Congress of the Communist Party of China, it aims to build biomedical science and technology capabilities with core competitiveness and independent intellectual property rights, and vigorously develop new-quality productive forces in biomedicine with Chinese characteristics. By promoting scientific and technological exchanges, integrating the entire industrial chain, and enhancing communication between government and enterprises as well as among industry, universities and research institutions, the congress will mobilize members of various associations and resources from all sectors of society to more actively engage in the historical process of "advancing the construction of Healthy China".
This congress invited experts, scholars and outstanding young scientists who have made remarkable achievements and exerted significant academic influence in the biomedical field. Centering on cutting-edge biomedical technologies, it set up a main venue and 11 parallel sessions covering topics including vaccine quality and R&D, recombinant therapeutic biologics, immune cells, stem cells and gene therapy, clinical research on innovative biologics, vaccination, blood products, international cooperation on vaccines, biopharmaceutical engineering technology, application of AI in the biomedical field, and digital transformation of biomedicine. Nearly 200 academic reports were organized during the event.
In addition, the congress featured a large-scale exhibition showcasing new biologics products, new technologies, new equipment, new materials, new facilities and various types of new service projects in the field. Nearly 300 leading domestic and foreign enterprises in the biomedical sector participated in the exhibition, which covered an area of 15,000 square meters.
During the congress, a variety of academic exchange, industrial exchange and industry exchange activities were held, comprehensively demonstrating the cutting-edge progress and innovative achievements of China's biomedical industry.






As a professional supplier that has served the biologics industry for 18 years, Beijing XMJ also exhibited a rich range of products at this congress. It provided one-stop product solutions for biopharmaceuticals, vaccines, and cell/gene therapy, which attracted many customers to stop and communicate, making the booth a lively spot.
The Lively Scene at the Booth






Beijing XMJ Scientific Co., Ltd. (hereinafter referred to as XMJ) was founded in 2006 by professionals in the biological field who returned to China after studying in the United States. It is a high-tech company integrating the sales of imported reagents, technical services and contract development, aiming to provide first-class products and services for the development of life sciences.
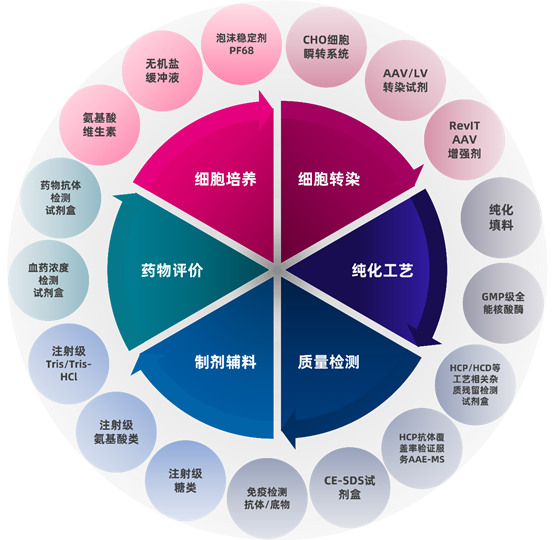
Over the 18 years since its establishment, XMJ has diligently and practically integrated high-quality global resources, and provided customers with a wealth of professional products and services. These include basic components of cell culture media, GMP-grade transfection reagents, various pharmacopoeia-grade biochemical reagents, purification fillers, GMP-grade nucleases, HCP/HCD detection kits, detection kits for residual impurities in bioprocesses such as Protein A/BSA/MVM, protein characterization analysis kits, injectable pharmaceutical excipients, antigens/antibodies/substrates, as well as applicability verification services for host protein residue detection kits and exclusive kit development services. These products and services meet the one-stop needs from R&D to production in aspects such as cell culture, transfection, purification process, quality control, preparation and drug evaluation.
XMJ holds import and export qualifications and has its own international logistics system. It has established long-term and stable cooperative relationships with more than 40 domestic and foreign suppliers of reagents, instruments, raw and auxiliary materials, and technical services, and has gathered dozens of well-known domestic and foreign brands including Applichem, Cygnus, Pfanstiehl, c-LEcta, Mirus, Astrea Bioseparations, KPL and ZF Biology. With the development of its business, XMJ has successively established branches in Shanghai, Guangzhou, Chengdu, Wuhan, Shenzhen and other cities. It has become a designated supplier for the procurement platforms of domestic biopharmaceutical enterprises, diagnostic enterprises, well-known multinational pharmaceutical enterprises, as well as major universities and scientific research institutions, and has won the support and trust of many customers.
For more information, please feel free to send email to info@xmjsci.com or visit the official website www.aoxiangbz.com.


 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號