BOUTIQUEResources
Affinity chromatography using Protein L is commonly employed to capture and purify the antigen-binding fragment (Fab), single-chain variable fragment (scFv), or single-domain antibody (sdAb) of antibodies—all of which are derived from the κ light chain of antibodies. Although Protein L is typically covalently bound to the chromatography matrix, it may still be eluted together with antibody fragments, becoming a process-related impurity that further affects the quality and safety of pharmaceuticals.
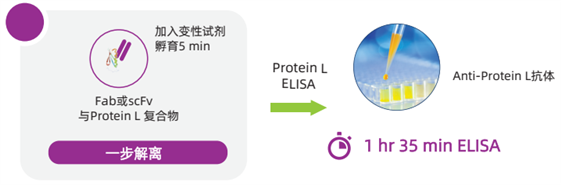
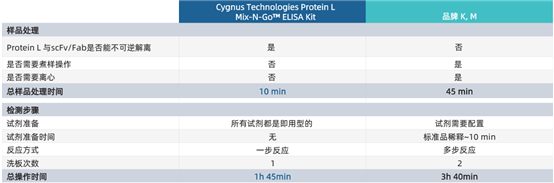
The Protein L Mix-N-Go? ELISA Kit developed and launched by Cygnus Technologies can accurately assess residual Protein L impurities in antibody fragment drugs. Since Protein L remains bound to the eluted antibody fragments after purification, this kit adopts a unique one-step sample processing method, which significantly simplifies the experimental procedures for Protein L detection, shortens the detection time, and improves the reliability of Protein L detection results compared with traditional ELISA methods.
Product Advantages
· No boiling or centrifugation steps required — reduces the use and calibration of equipment
· Robust method — ensures irreversible dissociation of Protein L from antibody fragment drugs
· Rapid access to test results — detection time is 2 hours shorter than that of other kits
· Higher precision — fewer steps lead to less error
· All reagents and pre-prepared standards required for detection are provided in the kit
·
One-Step Protein L Mix-N-Go? Sample Processing Procedure
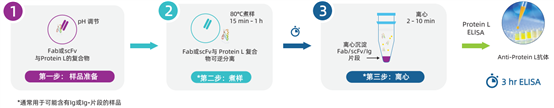
Sample Processing Steps for the Three-Step Conventional Protein L Detection Kit
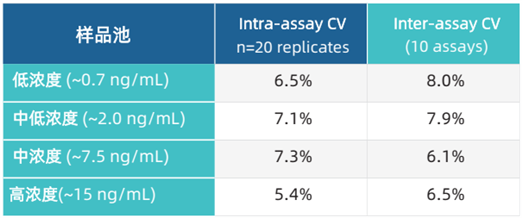
Precision Evaluation
Precision evaluation (including intra-assay and inter-assay precision) was performed on 4 sample pools with known concentrations.
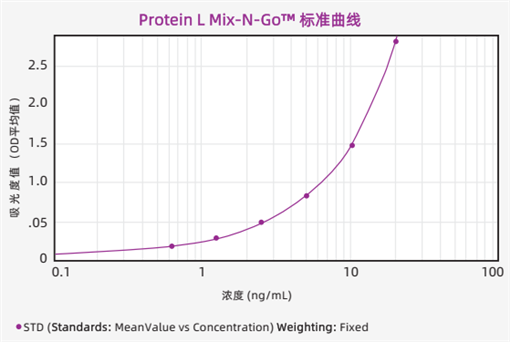
Standard Curve
The typical standard curve of the experimental data fitted by the 4-parameter method is shown in the figure below.
Streamlined Operating Procedure for the Protein L Mix-N-Go? ELISA Kit
Product Information for Protein L Mix-N-Go? ELISA Kit
· Catalog Number: F1065
· Storage Condition: 2°C ~ 8°C
· Specification: 96 Well Plate
· Operation Time: ~1 hr 45 min
· LOD: ~0.08 ng/mL
· LLOQ: ~0.63 ng/mL
· Recommended Sample Diluent Catalog Number: G028

Cygnus Technologies, LLC. provides products and analytical methods for the biotechnology and biopharmaceutical industries, aiming to accelerate the research and development (R&D) phase and enhance product quality. The bioprocess residual kits developed and manufactured by Cygnus are used to detect specific impurities from over 50 different expression systems. As an expert in high-sensitivity analytical technologies focused on immunoassays for biotechnological applications, Cygnus’ products and services have been utilized by nearly all major biopharmaceutical companies for more than 25 years.



.png) 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號