BOUTIQUEResources
In biopharmaceutical production, host cell protein (HCP) residuals are one of the key factors affecting drug stability. Among them, lipase-class HCPs are a major focus within HCP residuals due to their potential to degrade surfactants commonly used in formulations (such as Tween-80/20). Tween degradation leads to an increase in free fatty acids in the drug solution, which can induce protein aggregation, formation of fatty acid-protein complex particles, and subsequently impact product safety and efficacy.
While the ELISA HCP detection method can assess overall HCP levels, it cannot identify specific types of lipases, making it difficult to precisely evaluate process-related risks. To address this issue, Cygnus Technologies has developed a targeted proteomics analysis method based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) – the CHO 6xLipase? MS Assay. This method utilizes Parallel Reaction Monitoring (PRM) technology and Stable Isotope-Labeled peptides (SIL) to achieve absolute quantification of six CHO-derived HCP lipases known to cause Tween degradation::
|
HCP |
LOD (ppm) |
Quantification Range (ppm) |
|
Lysosomal Acid Lipase(LAL) |
0.15 |
18.25 - 292.06 |
|
Lipoprotein Lipase(LPL) |
0.08 |
0.10 - 323.34 |
|
Phospholipase A1(PLA1) |
0.12 |
0.49 - 311.67 |
|
Group XV Lysosomal Phospholipase(GXVPA2) |
0.03 |
4.72 - 302.31 |
|
Phospholipase B-Like 2(PLBL2) |
0.56 |
0.66 - 419.46 |
|
Phosphoinositide Phospholipase C(PIPLC) |
0.09 |
13.91 - 890.07 |
Technical Roadmap
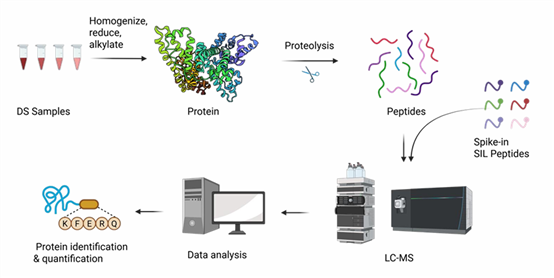
Technical Advantages:
? Pharmacopeial Method: PRM combined with SIL peptides is an absolute quantification method recognized by the USP <1132.1> guideline, with accuracy and precision far exceeding semi-quantitative methods.
? Excellent Linear Range: All 6 lipases show R2 ≥ 0.98, with a quantification range covering 0.1 - 890 ppm, meeting detection needs from early process stages to final product.
? Stringent Validation Standards: Accuracy error <35%, precision %CV <25%, Limit of Detection (LOD) as low as 0.03 - 0.56 ppm.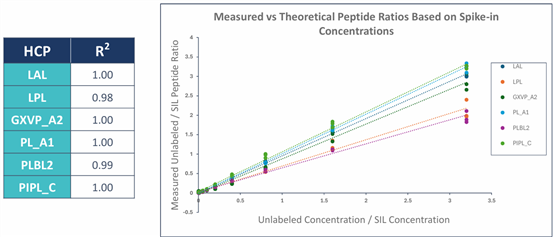
Client 1: High-Risk Sample (DS-top): Fc-Fusion Protein
? Tween20 degradation (complete degradation within 3 months)
? 5 lipases detected, multiple enzymes synergistically accelerating Tween degradation
|
HCP |
Predicted Concentration (ng/mL) |
%CV |
|
GXVP_A2 |
1.15 |
5.2% |
|
LAL |
2.76 |
9.6% |
|
LPL |
6.94 |
2.3% |
|
PIPL_C |
|
NA |
|
PL_A1 |
0.23 |
3,2% |
|
PLBL2 |
|
12.4 |
Client 2: Stable Sample (DS-bottom): Monoclonal Antibody
? Tween20 degradation detected
? Only PLBL2 detected (3.06 ng/mL), the other 5 were below the limit of detection
|
HCP |
Predicted Concentration (ng/mL) |
%CV |
|
GXVP_A2 |
|
NA |
|
LAL |
|
NA |
|
LPL |
|
NA |
|
PIPL_C |
|
NA |
|
PL_A1 |
|
NA |
|
PLBL2 |
3.06 |
3.20% |
Service Package:
- Standard Service Cycle: Approximately 6 weeks (from sample receipt to report issuance)
- Expedited Service: 4-week or 2-week options available (additional fees apply)
- Sample Requirement: 1 mL of DS sample quantified by BCA assay (concentration ≥ 4 mg/ml)
If you are interested in our lipase residual detection service, please click here to fill out the form. XMJ will contact you promptly to share more detailed information.

Cygnus Technologies, LLC. provides products and analytical methods for the biotechnology and biopharmaceutical industry, aiming to accelerate R&D stages and improve product quality. Cygnus develops and manufactures bioprocess residual kits for detecting specific impurities across over 50 different expression systems. As an expert in highly sensitive analytical techniques for biotechnology applications, particularly immunoassays, Cygnus's products and services have been used by nearly all major biopharmaceutical companies for over 25 years.

Beijing XMJ Science & Technology Co., Ltd., as the exclusive distributor of Cygnus in China, has established long-term and stable cooperative relationships with numerous renowned domestic pharmaceutical companies and CRO/CMO enterprises. Over the years, XMJ's products and services have helped many companies accelerate R&D stages, improve drug quality, purity, and safety, optimize R&D processes faster, reduce time-to-market, and lower QC costs. If you are interested in the above products, please feel free to contact XMJ customer service hotline at 400-050-4006 or visit the website www.aoxiangbz.com for more information.


 京公網安備 11010802028692號
京公網安備 11010802028692號